ANGIODIN PD 3000: New Version of Medical Equipment for Vascular Studies Under Registration Phase in Cuba
- Submitted by: admin
- Santiago de Cuba
- Business and Economy
- Health and Medicine
- Science and Technology
- 02 / 04 / 2008
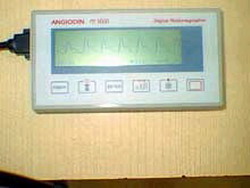
A new version of the ANGIODIN PD 3000 micro controlled digital plethysmograph, an instrument for the diagnosis of peripheral circulatory diseases currently operating in 25 hospitals in Cuba, entered the registration phase under the supervision of researchers from the Center of Medical Biophysics (CMB) in Santiago de Cuba.
"The new ANGIODIN version successfully passed the technical tests, increasing the diagnosis possibilities of the old version, which only detected arterial problems," said PhD. Manuel Arsenio Lores Guevara, director of the CMB, a prestigious institution that will turn 15 years next February 10.
The options of also carrying out studies in the venous system, and especially of determining key indicators for angiology, such as the venous reflux time or the arterial rigidity index as a risk indicator of cardiovascular accidents are, according to the expert, among the new features of the improved version.
"Accordingly," said Lores Guevara, "during the first semester of this year the CMB is about to undertake clinical tests to validate the advantages of the equipment, something that should lead to the registration of the new model by the end of 2008."
For more than ten years, the CMB has worked on the development of the equipment, its integration to the Cuban healthcare system and its commercialization abroad. The ANGIODIN PD 3000 uses plethysmography in the early and not invasive diagnosis of vascular diseases such as failure of peripheral arteries, diabetic micro and macroangiopathies, arterial vasospasm, arrhythmias and erectile sexual dysfunction of physical origin, among others.
The effectiveness of the equipment, its great autonomy for carrying out the tests, and the possibility of using it as a portable device in health units, validate the introduction of plethysmographs to the vascular hemodynamic laboratories of the main hospitals in Cuba, as well as the installation of some 120 of these units in healthcare institutions in other countries, such as Switzerland, Peru and Venezuela.
The National Institute of Angiology and Vascular Surgery also recommended the use of ANGIODIN PD 3000 as the basic technology to evaluate the effectiveness of new Cuban products such as the CITOPROT P, which is in phase three of the preclinical tests in 20 centers throughout the country.
Source: Vanguardia Newspaper (Translated Cubaheadlines Staff)
Comments